The Premier Urology Group in the Inland Empire
Welcome to
San Bernardino
Urology Associates
Providing excellent, cutting edge, and compassionate urologic care to the community that we serve.
Experienced specialists
Men's
Health
Specific needs of
Women's
Health
Advanced
Urologic
Cancer
Clinic
World class surgeons
Robotic
Surgery
Institute
About Us
The Highest Quality Care
Delivered with Compassion
Since 1985, San Bernardino Urology Associates has established itself as the leading urologic practice serving the Inland Empire and High Desert.
Our compassionate doctors have undergone subspecialty training and are highly regarded for their advanced and minimally invasive robotic, endoscopic, and laser surgical techniques.
We aim to use these state-of-the-art diagnostics and advanced treatment techniques to deliver personalized care tailored to meet your specific needs.

Robotic Center of Excellence
World Class Precision Surgery
Minimally invasive surgery maximizes visualization, precision, and control of instruments allowing for the potential to minimize the risk of complications, shorten healing time and enable safer, more precise surgery.
Combined with the most experienced fellowship trained robotic surgeons in the Inland Empire, patients will experience the best possible robotic surgery for their cancer or other urologic condition.
We are proud to announce that SRC, an independent safety organization, recognizes our surgeons as “Surgeons of Excellence in Robotic Surgery.”
Common Conditions We Treat
Benign Prostatic Hyperplasia (BPH) is a condition in which the prostate enlarges as men get older causing bothersome urinary symptoms.
Kidney stones are hard deposits of minerals and acid salts that stick together in concentrated urine.
Prostate cancer is the most common non-skin cancer among men, and about 170,000 new cases of prostate cancer will occur this year.
Incontinence affects twice as many women as men, which is believed to be due to pregnancy, childbirth and menopause.
ED means difficulty achieving, sustaining erections rigid enough for sex. It affects more than 3 million men a year in the U.S.
- Why Choose Us?
- 1. Clinical Research Center: Providing Cutting Edge Urological Care (link to the Clinical Trials)
- 2. Compassionate and Experienced Doctors (link to the doctors section)
- 3. Comfortable Office Based Procedures (link to the pronox section)
Why Choose Us?

Clinical Research Center:
Providing Cutting Edge Urological Care

Compassionate and Experienced Doctors
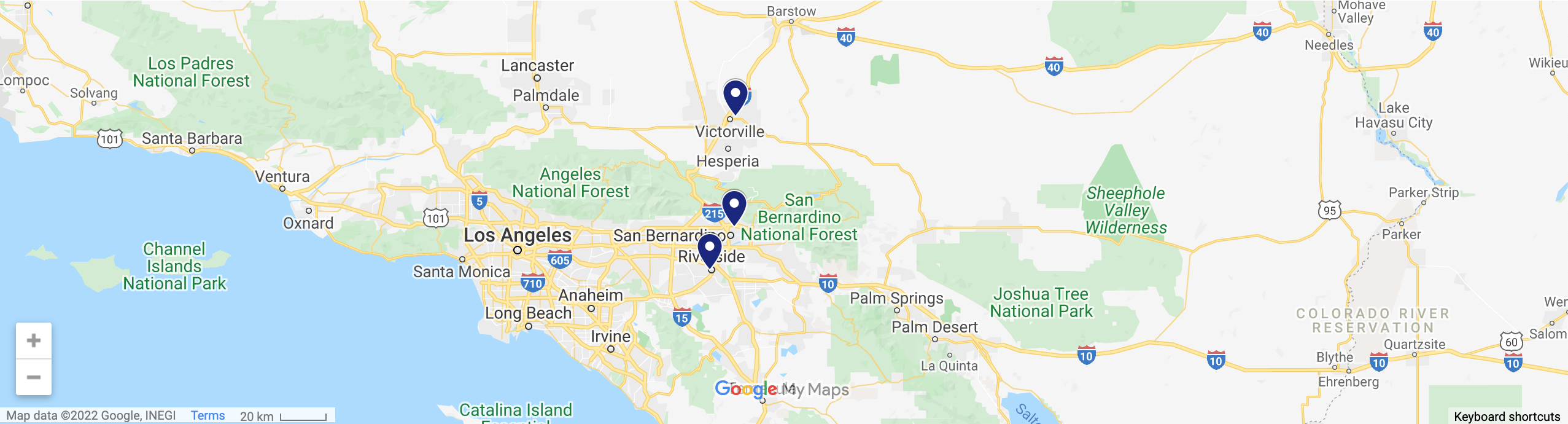
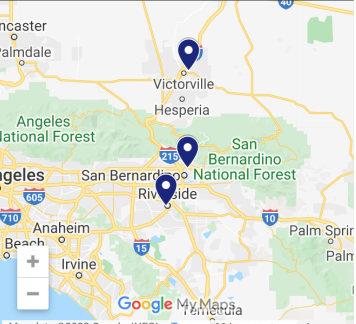
Three Locations To Serve You
Phone: (760) 242-6576
Address: 15995 Tuscola Road, Suite 204, Apple Valley, CA
Phone: (909) 882-2973
Address: 489 E 21st Street, San Bernardino, CA 92404
Phone: (951) 213-6767
Address: 4440 Brockton Ave, Suite 210, Riverside, CA
Phone: (760) 242-6576
Address: 15995 Tuscola Road, Suite 204, Apple Valley, CA
Phone: (909) 882-2973
Address: 489 E 21st Street, San Bernardino, CA 92404
Phone: (951) 213-6767
Address: 4440 Brockton Ave, Suite 210, Riverside, CA